Prostaglandin Analogues
There are four main groups of IOP lowering eye-drops that are in common use. This list of options has expanded greatly over the past 20 to 25 years giving the clinician quite a range of options before needing to consider surgical options for patients. They work either by reducing aqueous production or increasing drainage. Often a combination of agents is required – with a lot of these agents being available in fixed combinations with each other.
In more recent years there has been an expansion in the number of drops available in preservative-free options which in certain situations can reduce the side-effect risks and aid compliance. The first of these groups we will look at are the Prostaglandin Analogues.
Prostaglandin analogues were developed in the late 1990’s and have really become established as the first line of treatment. They combine a good level of IOP lowering with a low side-effect profile and ease of use as they are used just once daily.
They work by a different mechanism to most eyedrops by lower IOP through enhanced drainage of aqueous through the uveo-scleral pathway.
This exact mechanism isn’t fully understood – however we often see the IOP being lowered by 25-30% from baseline, which is a significant response.
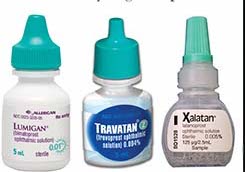
There are four main drugs in this group – bimatoprost, latanoprost, tafluprost and travaprost. They often appear in combination products with beta-blockers and depending on the drug can be with or without preservatives.
Possible side effects with PGAs
Prostaglandins are chemical mediators which occur throughout our bodies with a wide range of effects on different tissues. Some are involved in the inflammatory response, so then these drugs were first launched, there was a concern that they may induce uveitis in patients, particularly following surgery, however the evidence since then has never really proven this to be the case. Similarly there were concerns about Cystoid Macular Oedema developing more readily in patients on these drops, however again this hasn’t really played out. It Is thought that there is a higher risk of CMO in aphakic patients or those where the posterior capsule has been breached.
There have been some case reports of Herpes Simplex Keratitis being reactivated with PGA use, but again this is a significant trend. Any of these complication resolve when the PGA has been ceased.
There are a few local side effects to the eyes. Prolonged PGA use can cause changes to iris colour.
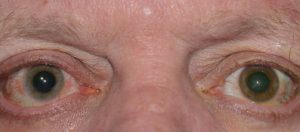
Around 10-18% of patients are affected in the first 2 years of treatment, and those over 75 are at higher risk. Irises which are made up of a mixture of colours such as hazel/green eyes tend to be affected more readily, against those eyes which are very uniformly blue or brown. This is thought to be due to a build up of melanin causing a darkening of the iris. It is worth pre-warning patients about this possible change, especially those being treated in just one eye. It is also worth noting that these changes are irreversible.
The other pigmentary change that can occur is a peri-ocular darkening of the skin, again due to changes in melanin. This is thought to occur in around 3% of patients on these drops and in this case the changes do reverse once the drop has been stopped.

The third and possibly most common change these patients can develop is in their eye/lashes. Around 50% will notice eye lash growth increasing the length, thickness, number and curvature of their lashes.
One general side effect I have encountered with two patients who I have treated with PGAs, is that of developing a skin rash. The reason for this is unclear possibly due to some inflammatory process. In both cases the rash cleared after the medication was stopped.
These drops should be avoided during pregnancy as certain prostaglandins have an affect on uterine muscle tissue, however the concentration of this drop in the bloodstream would be incredibly low, however best not to take any risks.
As with all drops these can cause ocular redness, hyperamia and watering if the eyes are sensitive to the drug or any of the other ingredients, particularly if preserved with benzalkonium chloride.
